PRESENTATION
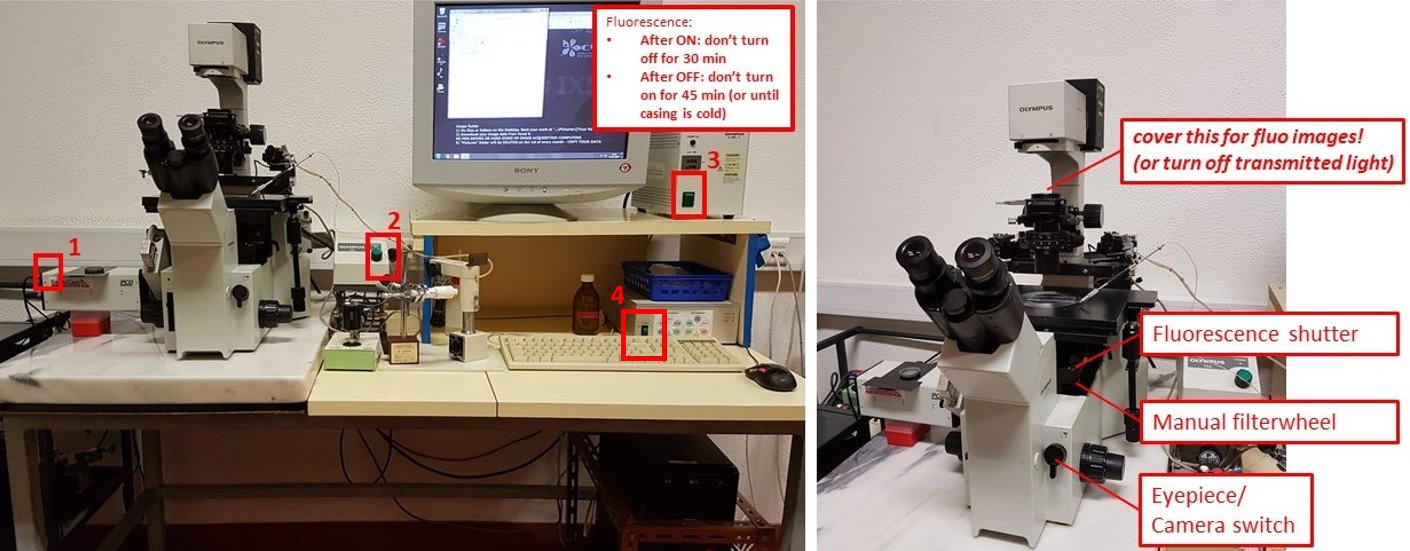
Olympus IX50 manual fluorescence microscope with mercury fluorescence illuminator, Nomarski/DIC Prism, cool CCD for fast low-light imaging, motorized filter wheels and micromanipulation / microinjection
Room: 2.1.15
IP: 10.101.134.37 (why?)
Check “What are the costs” at the Users section
| Pos | Mag | NA | IM | Corr. | WD (mm) | Glass (mm) | Color Code | Obs |
|---|---|---|---|---|---|---|---|---|
| 1 | 10x | 0.40 | Air / Water | UPlanApo | 3.10 | - | - | |
| 2 | 20x | 0.20 | Air | LCPlanFL | 7.55 - 6.75 | - | - | |
| 3 | 40x | 0.85 | Air | UPlanApo | 0.20 | - | - | |
| 4 | 100x | 1.25 | Oil | PlanC N | - | - | - |
| Position | Excitation (nm) | Emission (nm) | Fluorophores | Model |
|---|---|---|---|---|
| 1 | BP 330-385 | LP >420 | DAPI, hoechst | |
| 2 | BP 470-490 | LP >515 | GFP, Alexa488, cy2, FITC | |
| 3 | BP 530-550 | LP >590 | RFP, YFP, Alexa546, Alexa568, cy3, TRITC | |
Transmitted light
Halogen lamp
Fluorescence
Olympus U-RFL-T Mercury lamp
PCO Sensicam-QE 12bit
Ludl filter wheels for 6 excitation and 6 emission filters
Narishige micromanipulator
BASIC PROCEDURE FOR MULTICHANNEL GRAYSCALE IMAGES
- Turn on PCO SensiCam camera (button 1).
- Turn on transmitted light source box (button 2)
- If doing fluorescence imaging, turn on fluorescence source box (button 3). Do not switch it off for 30 minutes after switching on, and DO NOT switch on if the metal casing is still warm and it was switched on less than 45 minutes before.
- Turn on motorised filterwheels control box (software will give out an error without it)
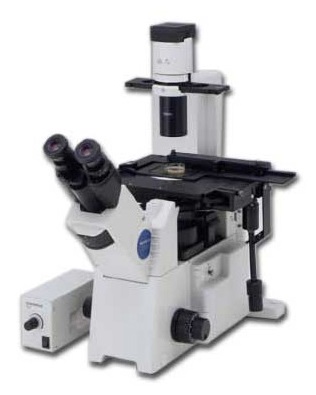
- Turn the Eyepiece/Camera knob to eyepieces, rotate the Fluorescence shutter to open, and select desired filtercube with the manual filterwheel. Select desired objective and bring your sample into focus.
- Turn on the acquisition PC and start uManager program
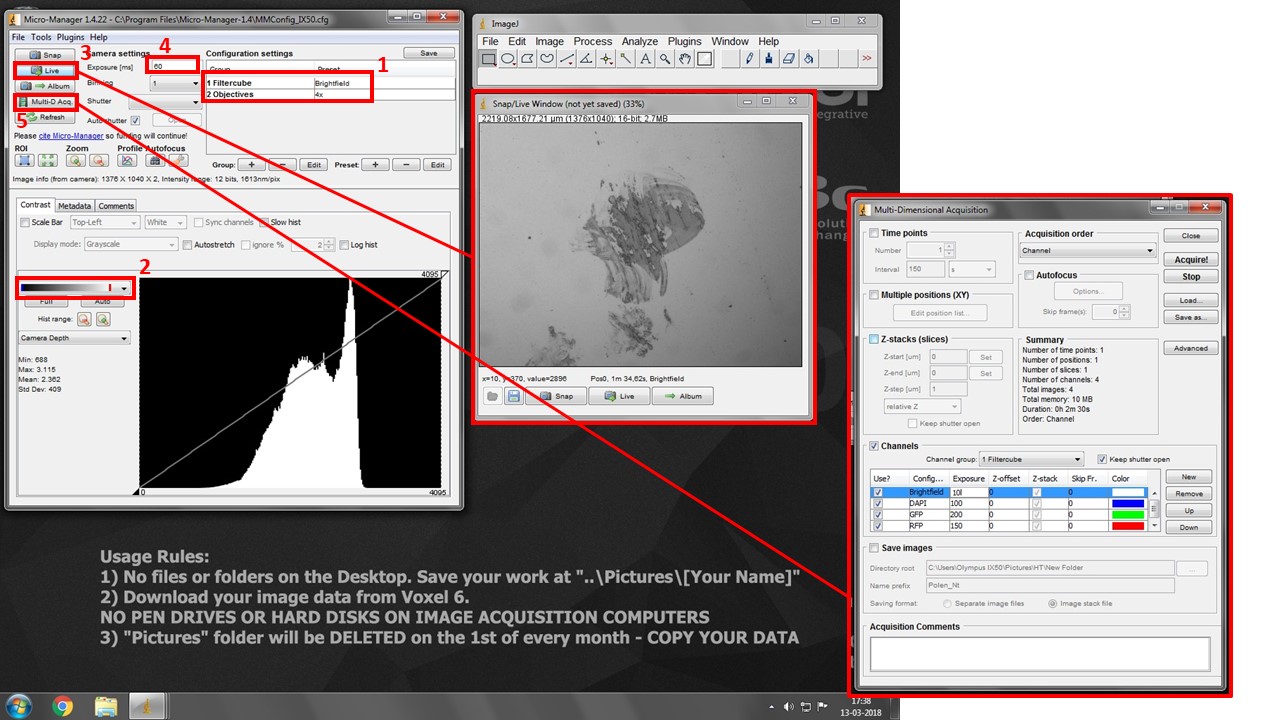
- Turn the Eyepiece/Camera knob to camera position.
- Select “Open” position in both Excitation and Emission Filterwheel drop-down boxes. This is the only motorised setting controled by the software.
- Select both desired filtercube and objective in the drop-down boxes in box (1). THESE ARE SOFTWARE SETTINGS AND DO NOT CONTROL THE MICROSCOPE. But they do allow you to set different exposures for each channel for multidimensional acquisition, and snap spatially calibrated images for each objective.
- Pick the second lookup table in box (2). It will show black pixels as BLUE and white pixels as RED. This will help you with exposure settings.
- Press “Live” button (3) to see your image.
- Increase exposure (4) in each filter combination (change to the adequate “Filtercube” in (1) each time) until you see your signal, but very little to no saturation (white/red pixels).
- Press Multi-D. Acq. Button (5) and check “Channels”. Add desired channels, pick a suitable lookup table color for each, and insert previously set Exposure values.
- Press “Acquire” to start multi-dimensional acquisition. Turn manual filterwheel as requested by the software, and minimize table vibrations as the software is acquiring images.
- Click on the little “settings gear” icon at the bottom right of the acquired image and click “Send to ImageJ”. It will create a proper multichannel image for saving.
- Go to ImageJ File > Save As > save as TIFF. You will be saving a composite image with “x” 12/16-bit greyscale channels. Save it on your image folder. Copy your images from Voxel 6.
- Turn off system in reverse order.
This system is equipped with DIC and two motorized filterwheels which can be enabled for fine fluorescent spectra adjustment. Contact Luís for help setting this up.
The system is also equipped with Narishige micro-manipulators/injectors. Contact Prof. Rui Malhó for instructions on setting up these kinds of experiments.
uManager for semi-automated multi-dimensional acquisition and control of filter wheels.
(Deprecated) Image Pro-Plus with Scope-Pro (control of filter wheels) and Sharpstack (deconvolution module)
LINKS / USER MANUALS
PEOPLE RESPONSIBLE FOR THE EQUIPMENT
 |
 |
 |
| Rui Malhó
FCUL / BioISI Head of Microscopy Facility r.malho@fc.ul.pt Room 2.1.44 |
Telmo Nunes
FCUL Microscopy technician mevarrimento@fc.ul.pt Room 2.1.15 |
Luís Marques
FCUL Microscopy technician lfmarques@fc.ul.pt Room 8.1.79 / 2.1.15 |