Inverted motorized fluorescence microscope for High-Throughput Screening
The Leica DMI6000 B is a fully automated inverted fluorescence microscope. It features automated contrast and illumination manager, motorized Z focus, automatic brightness and diaphragm adjustment, autofocus, automated multiposition imaging and other automated functions. This system is suitable for high throughput projects and automated imaging in multiwell imaging media. Temperature/CO2 control is available.
Room 8.1.79
Computer name: leicadmi6000b / 10.101.158.82 (why?)
| Position | Magnification | Numerical Aperture | Immersion Medium | Correction | WD (mm) | Glass (mm) | Color Code | Observations |
|---|---|---|---|---|---|---|---|---|
| 1 | 5x | 0.15 | Air | PL S-APO | 13.7 | - | DIC | |
| 2 | 10x | 0.40 | Air | HC PL APO | 2.2 | 0.17 | ||
| 3 | 20x | 0.75 | Water, glycerol, oil | HC PL APO | 0.68 | - | Correction collar | |
| 4 | 40x | 1.10 | Water | HCX PL APO | 0.63 | 0.14-0.18 | Correction collar | |
| 5 | 63x | 0.7 | Air | HC PL FLUOTAR L | 2.6 | 0.1-1.3 | DIC | |
| 6 | 20x | 0.55 | Air | HC PL FLUOTAR | 1.2 | 0.17 | DIC |
No phase contrast; DIC ON 5x, 20x, 63x
| Position | Excitation (nm) | Emission (nm) | Fluorophores | Model |
|---|---|---|---|---|
| 1 | 340-380 | 450-490 | DAPI, hoechst | A |
| 2 | 425-445 | 455-485 | CFP | custom CFP |
| 3 | 490-510 | 520-550 | GFP, Alexa488, cy2, FITC, YFP | YFP |
| 4 | 515-560 | >590 | mCherry, cy3, TRITC, Alexa568 | N2.1 |
| 5 | 630-660 | >670 | Alexa 633, Alexa647, cy5 | custom Cy5 |
| 6 | - | - | - | DIC ANA + BF |
Transmitted light
Halogen lamp
Fluorescence
Mercury metal halide bulb
Lifetime: 2000 hours
Integrated fast shutter
Monochrome CMOS camera 4.0 MP (2048 x 2048 pixels)
Pixel size 6.5 μm × 6.5 μm
Bit depth 16 bit
Peltier cooling
Water immersion micro dispenser
Bartels Mikrotechnik Extended Micropump Controller
Allows continuous water distribution for automated imaging of multiwell plates. Available for all water immersion objectives (20x and 40x).
Universal supports for for multiwell plates and microscopy slides
Immersion oil (RI 1.5180)
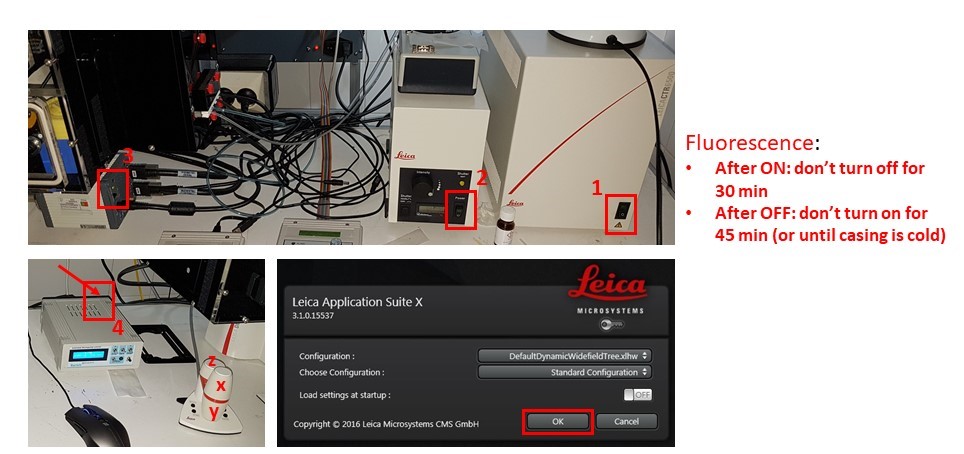
- Turn on microscope controller box (1), fluorescence source if needed (2), Hamamatsu camera (3), and immersion pump (4). Do not switch fluorescence source off for 30 minutes after switching on, and DO NOT switch on if the metal casing is still warm and it was switched on less than 45 minutes before.
- Allow motorized stage to finish full range motion for calibration. Start LAS X software, and click “OK” on the configuration window.
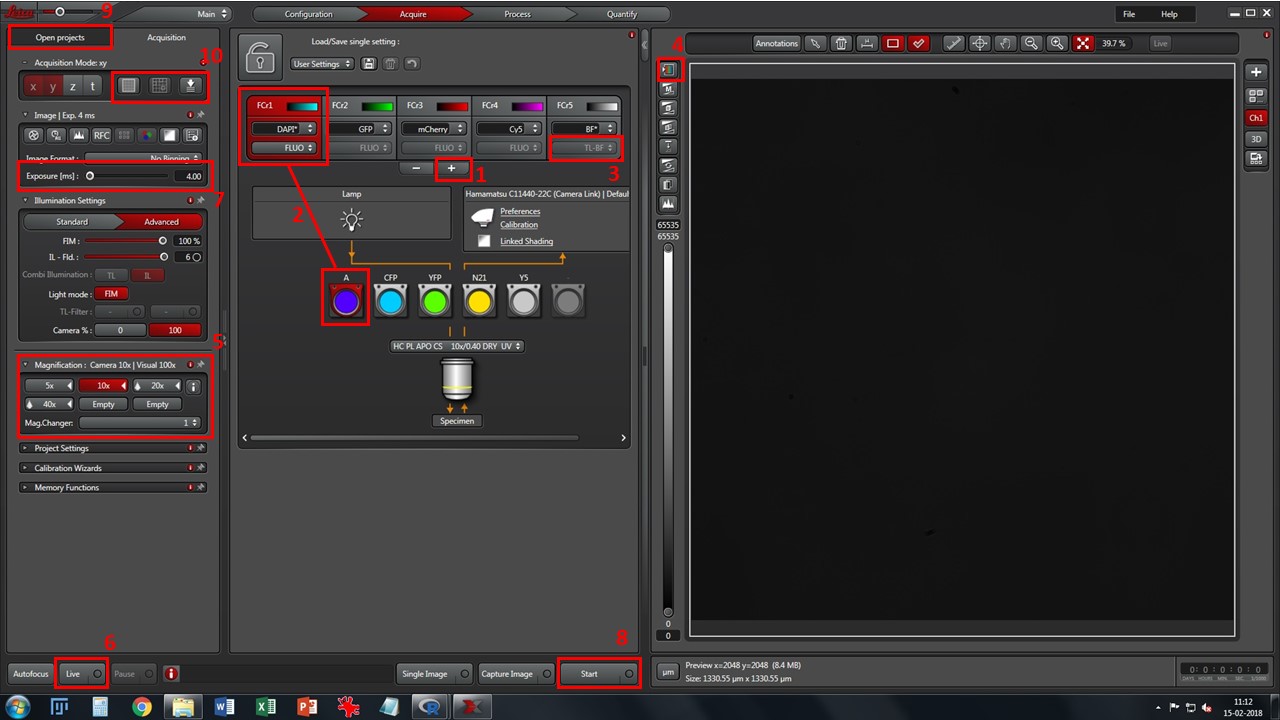
- Add desired fluorescence channels using button 1, and match each one to the appropriate filtercube (2). Change to preferred lookup table color in each one by clicking in the colored window. If using brightfield, select TL-BF instead of FLUO from the drop-down box (3)
- Cycle through viewing lookup tables (button 4) and pick the “saturation” one. Grey levels will appear as reddish-brown, black pixels will show in green and white pixels will show in blue. This will help you choose proper exposure for each channel.
- Select a low magnification objective (5) – 10x is a good starting point. Click one of the channels (preferably one with good signal, usually nuclei stained with DAPI/Hoechst), press the “Eyepiece” selector button on the microscope console, and then the “Shutter” button. Look through the eyepieces, move through your slide with the X-Y-Z joystick, and bring your sample into focus.
- Press “Live” button (6), and adjust proper exposure for each channel (7). Avoid white/saturated pixels which show up in blue in the viewing window.
- When all channels are set up with the proper exposure, press “Start” button (8) for acquisition. If you change objective, readjust exposure.
- Your acquisitions will be gathered as *.lif files, in the “Open projects” tab (9). Save it in your image folder. You may create “collections” inside *.lif files by right-clicking on them. A typical structure may be [Project: Experiment name or Date] \ [Collection: slide name or number] \ [Image: name of experimental condition]
- When you’re done with work, check if there are users scheduled after you. If not, close program, switch off machinery in reverse order, and leave a notepad.exe note on the computer desktop informing the time you switched off the fluorescence.
This microscope allows stage experiments (mosaic, mark&find) and automated fine focus (box 10). Contact Luís for further help with these settings.
Live imaging (with temperature and CO2 control) is also available, as well as High Content Data acquisition. Contact Hugo or Luís for assistance in setting up this kind of experiments.
Including Leica High Content Screening Software (MatrixScreener)
BioISI users: 0 €/h
External users with BioISI collaboration: 10 €/h
External users: 15 €/h
EQUIPMENT RESPONSIBLE
 |
 |
| Hugo Botelho
hmbotelho@fc.ul.pt Room 8.1.76 |
Luís Marques
FCUL Microscopy technician lfmarques@fc.ul.pt Room 8.1.79 / 2.1.15 |

